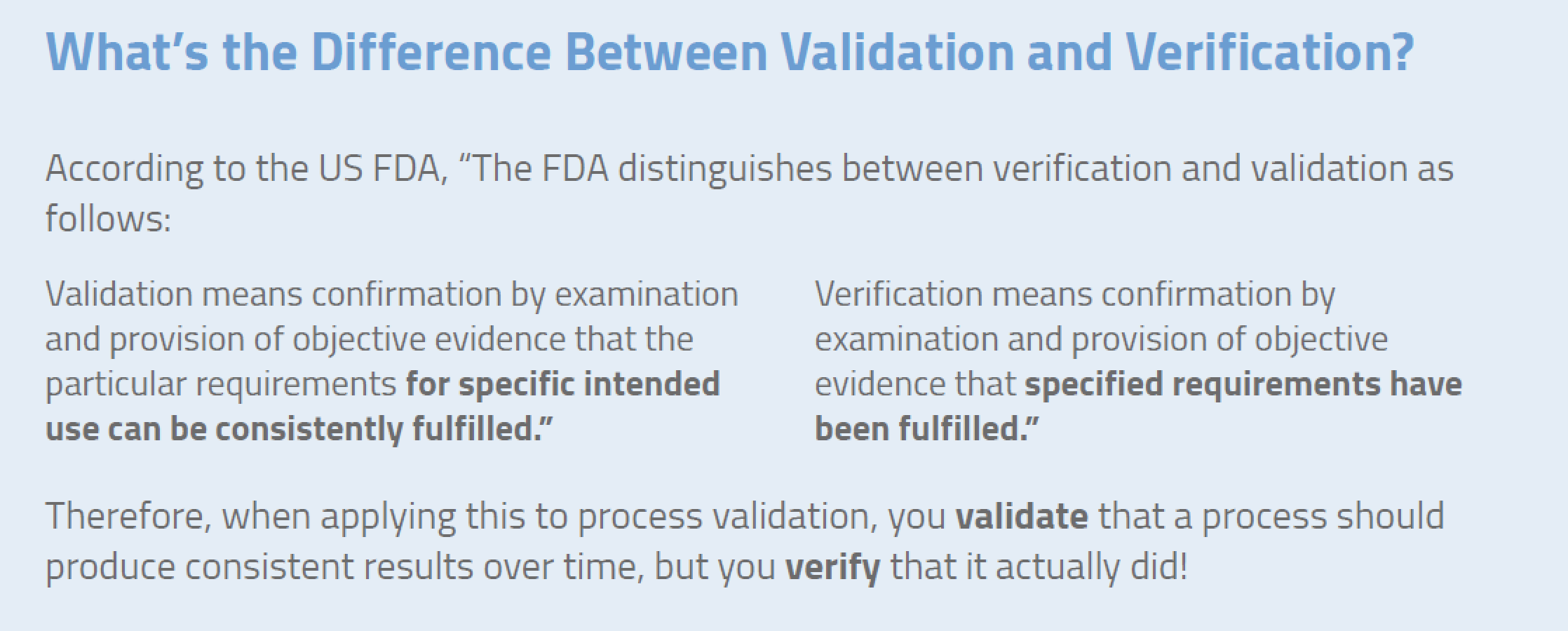
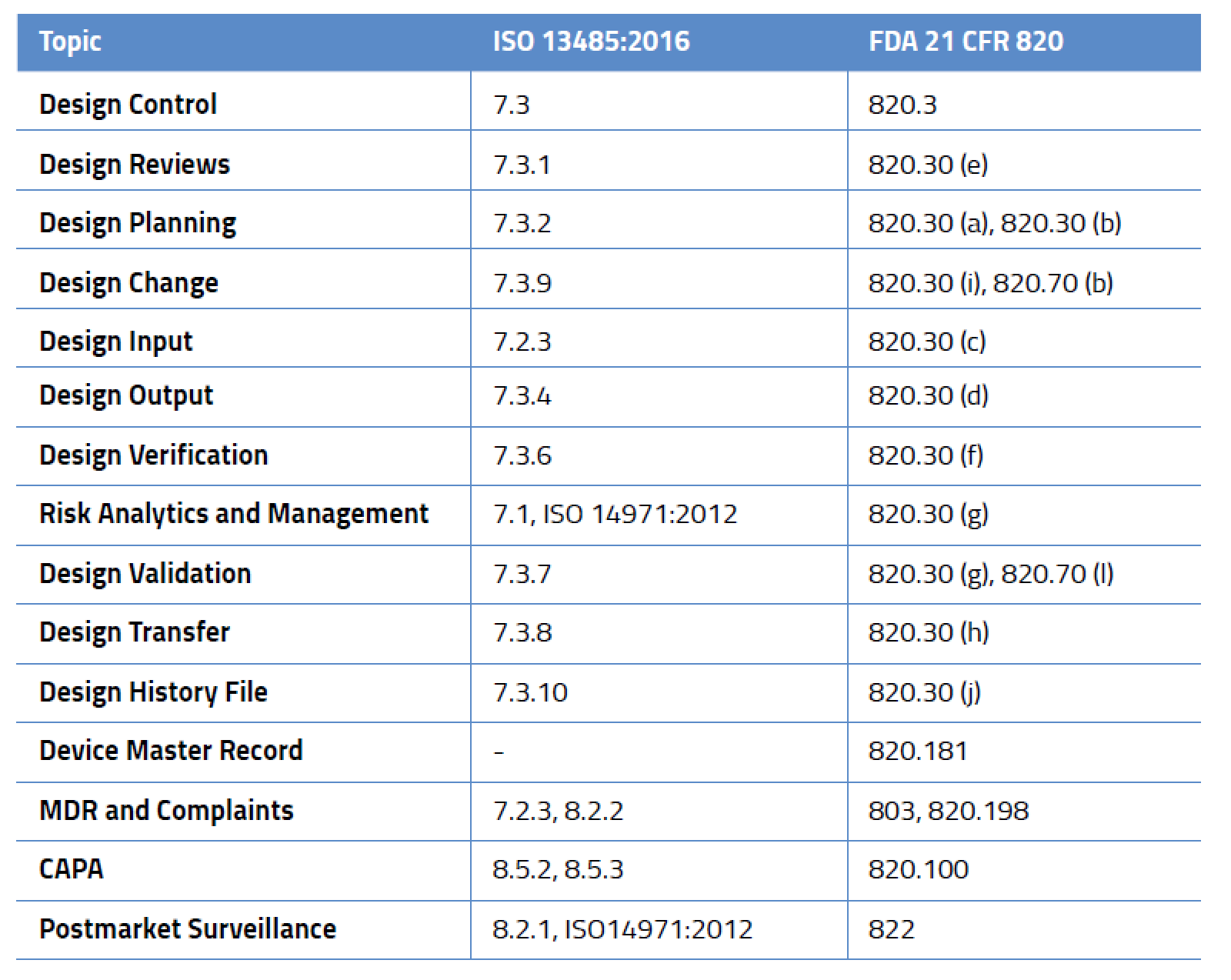
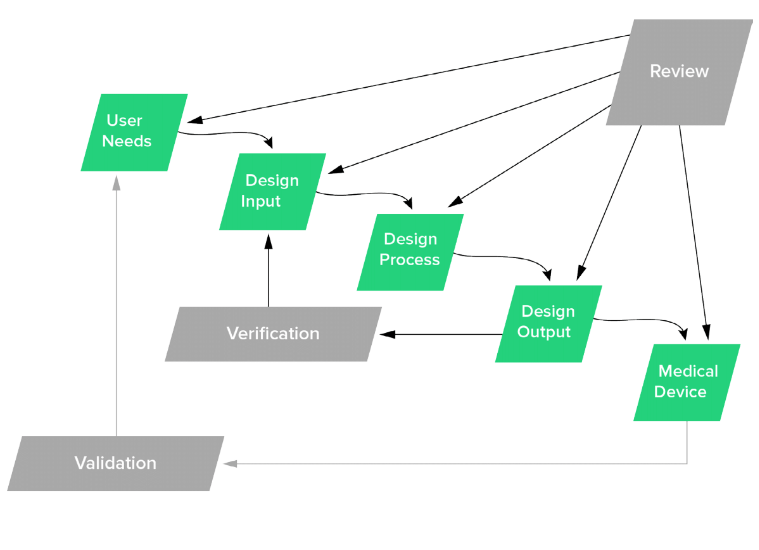
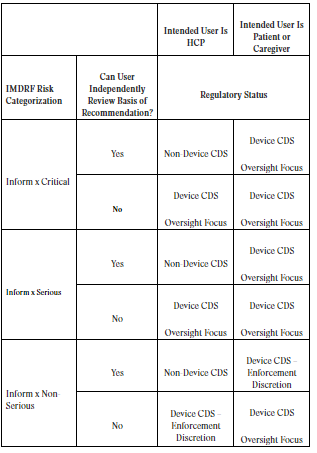
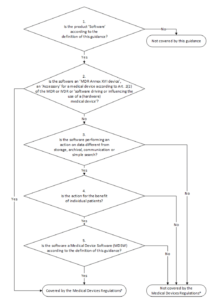
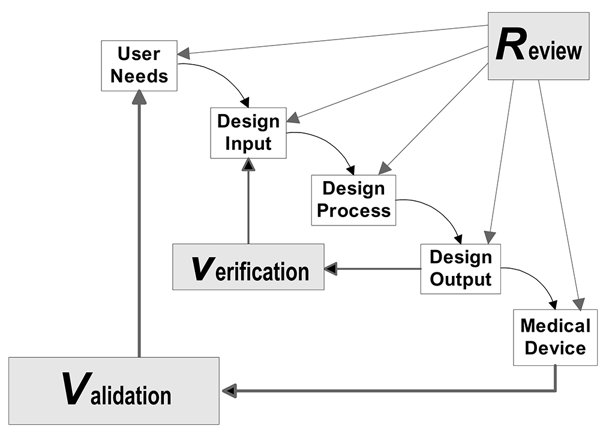
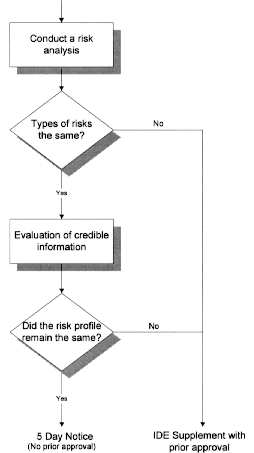
Legal Manufacturer Definition Medical Devices Fda

The natural or legal person who is responsible for the design manufacture packaging and labeling of a device before it is placed on the market under his own name regardless of whether these operations are carried out by that person himself or on his behalf by a third party.
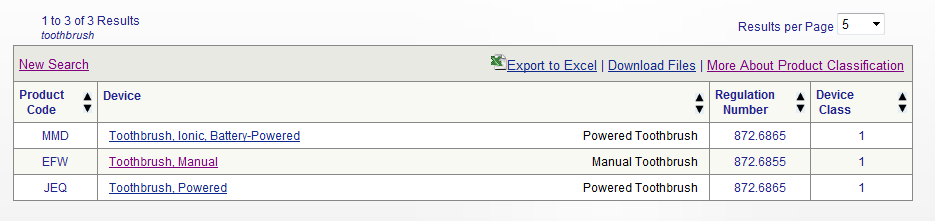
Legal manufacturer definition medical devices fda. T manufacturer means any legal person or entity engaged in the manufacture of a product subject to license under the act. Introduction to medical device labeling label vs. The medical device reporting mdr regulation 21 cfr part 803 contains mandatory requirements for manufacturers importers and device user facilities to report certain device related adverse. 1040 et seq as amended 21 u s c.
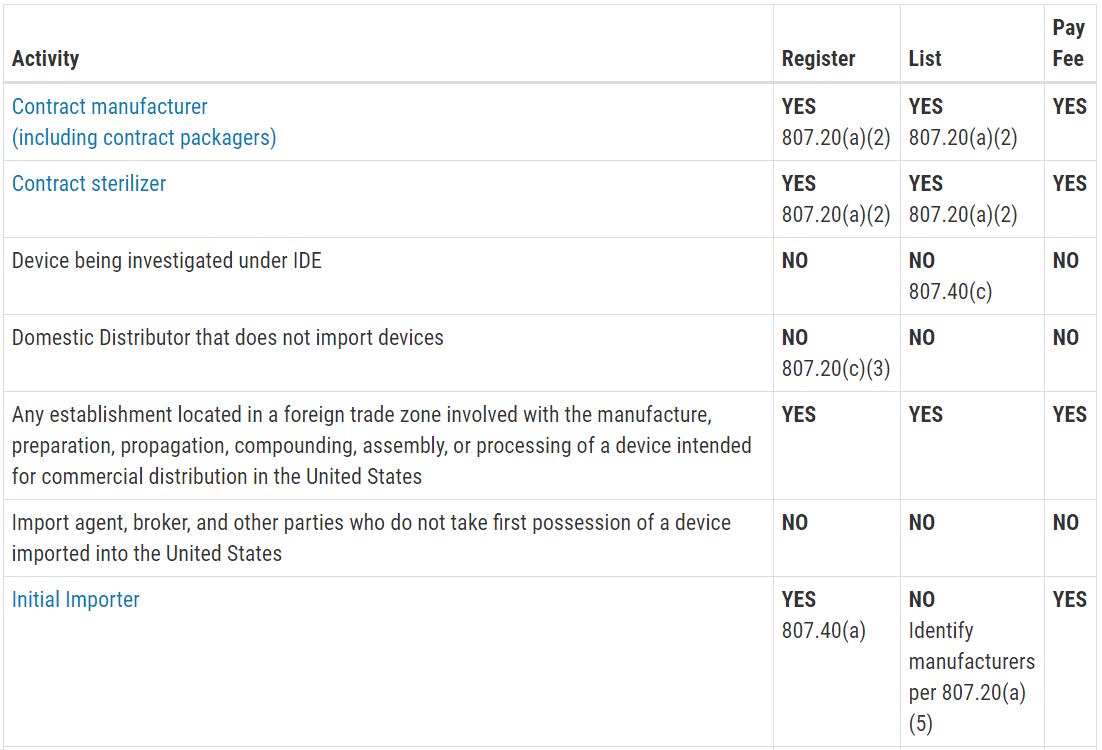
Manufacturer also includes any legal person or entity who is an. B complaint means any written electronic or oral communication that alleges deficiencies related to the identity quality durability reliability safety. A act means the federal food drug and cosmetic act as amended secs. Legal manufacturer means the manufacturer of a medical device in the meaning of the german medical devices act hersteller directive 98 79 ec and other applicable laws i e.
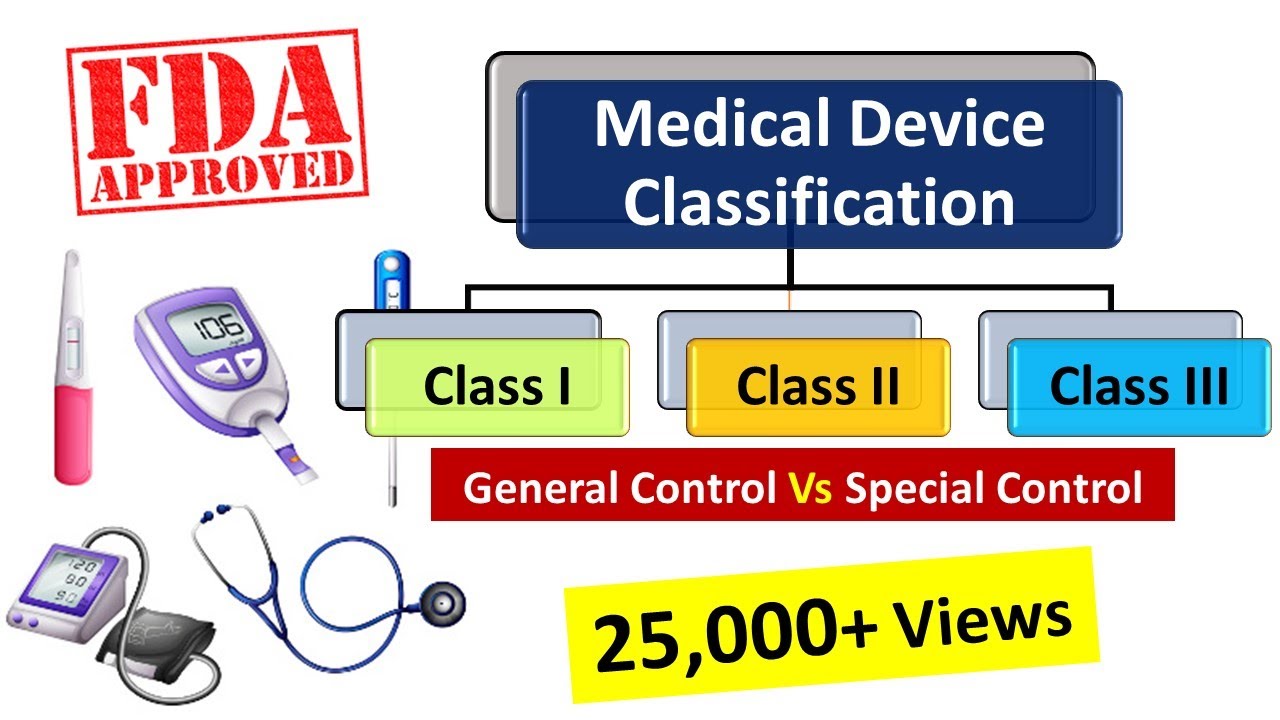
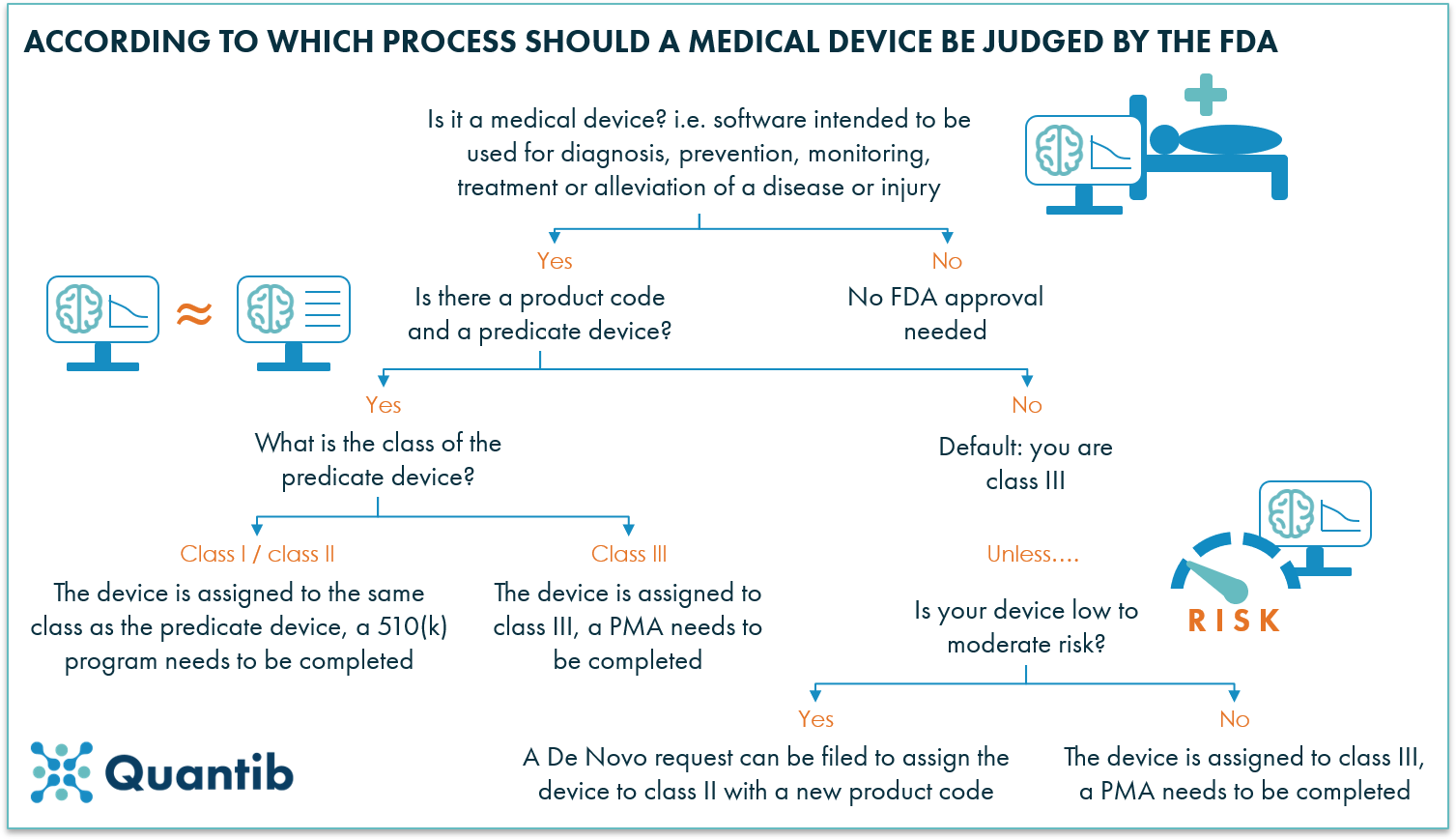
All definitions in section 201 of the act shall apply to the regulations in this part. Food and drug administration fda develops and administers regulations under authority granted by laws passed by congress that. Manufacturer makes by chemical physical biological or other procedures any article that meets the definition of device in section 201 h of the federal food drug and cosmetic fd c act.